The following information was updated in the Lab Catalog.
Test Utilization
Outpatient Respiratory Viral Testing
Spectrum Health Lab has noticed an increase in outpatient orders for Respiratory Pathogens by Film Array [LAB3359]. This may be due to a shortage of supplies for some Point of Care (POC) testing platforms. Film Array is typically used for emergency and high acuity patients and therefore has a high cost that may not be covered by most patients’ insurance. To lesson out of pocket costs for your patients, please order the below for RSV, COVID, or Flu testing.
The mitigation strategies enacted during the COVID-19 pandemic to reduce the spread of this virus have also impacted the transmission of other respiratory viruses. Influenza is typically prevalent during the winter months (December through March), however, influenza cases were essentially non-existent during the 2020-2021 season and influenza is not currently circulating in our community.
In the absence of circulating influenza activity, it is recommended to use the most sensitive diagnostic test in order to obtain accurate and actionable results. Influenza antigen testing (e.g. Sofia instrument) is not appropriate at this time. More detailed information can be found as published by the CDC: Algorithm to assist in the interpretation of influenza testing results and clinical decision-making during periods when influenza viruses are NOT circulating in the community
| Low Influenza prevalence |
High influenza prevalence |
|
| Recommended order | Influenza PCR (LAB3255) | Influenza Rapid Antigen (LAB2111530) or Influenza PCR (LAB3255) |
TEST INFORMATION
| Test name | Epic code | Interface EMR Code | CPT Code |
| Influenza PCR | LAB3255 | 11594 | 87502 |
| Influenza Rapid Antigen | LAB2111530 | 11208 | 87804 x4 |
Effective September 15, 2021, Spectrum Health’s Epic will contain new ask-at-order questions to help improve clinical decision support and appropriate utilization of stool ova and parasite (O&P) testing. O&P testing may be used to diagnose several parasitic infections, though the staining of stool smears and their microscopic review is very labor intensive for laboratories. Historically, O&P testing has been widely ordered for patients with diarrhea, however, there are now other testing options able to detect the most common pathogens associated with community-acquired diarrhea. While Enteric Pathogens PCR and Giardia/Cryptosporidium testing is more appropriate for the identification of common bacterial/viral and parasitic pathogens, respectively, O&P testing should be reserved for patients with specific exposure or immune status criteria.
The Pharmacy and Infectious Disease Stewardship Committee has endorsed the following order criteria for O&P testing. If any of these criteria are met, then then order can be placed.
♦ Past foreign residence or recent foreign travel followed by at least 2 weeks of diarrhea.
♦ Immunocompromised status.
♦ Unexplained microcytic anemia or peripheral eosinophilia.
♦ Unique exposure (daycare, MSM, waterborne outbreak, etc.)
Note: If no criteria are met, cancel the order and consider Giardia/Cryptosporidium Screen (LAB258) or Enteric Pathogens PCR testing (LAB3618).
Please direct question to the Contact Us link above.
TEST INFORMATION
Ova and Parasites, Complete – Epic: #LAB9550, Interface Code #50033, CPT #87328, 87329, 87177, 87209
Enteric Pathogens by PCR – Epic #LAB3618, Interface Code #55078, CPT #87506
Giardia/Cryptosporidium Screen – Epic #LAB258, Interface Code #50025, CPT #87329, 87328
Reminder: COVID Orders and Scheduling
Effective July 1, 2021, Spectrum Health no longer offers patient self-screening through MyChart or telephonic COVID screening appointments to patients. Patients will need an order from their provider prior to scheduling a COVID-19 test. Patients should be symptomatic or have a recent exposure, all other visit types should go through Michigan.gov (travel, athletic, or return to work or school, etc.)
If the patient does not have a provider, they may utilize the SHNow On Demand Visit via MyChart or find testing site options on Michigan.gov.
Once the order is placed, patients may schedule an appointment through MyChart or calling the Covid Hotline (for paper orders).
For more information:
Coagulation Blue Top Tube Shortage
Update 9/1/2021:
September 1, 2021
There continues to be a global shortage of sodium citrate (“blue top”) blood collection tubes used for coagulation testing as a result of unprecedented demand, in part due to COVID-19 surges, vaccine and treatment development. This was anticipated to last through August 2021, but now, due to continued unprecedented demands, there is no end date in sight. Please take this into consideration when ordering coagulation testing (i.e. Protime, aPTT, Fibrinogen, D-dimer, Lupus Screens, Factor Assays, Mixing Studies, von Willebrand testing, etc.).
As of August 26, 2020, the following tests will be obsoleted or made as lab orderables only. This was implemented to streamline protein electrophoresis orders and ensure that patients receive the recommended testing for the assessment of plasma cell proliferative diseases.
In addition to streamlining protein electrophoresis orders, order questions have been implemented to the available tests to access the reason for ordering to ensure that the proper reflex testing is applied.
For more information, see the lab test directory under the available tests for a protein electrophoresis orders tip sheet entitled “Orders- Protein Orderable Changes”
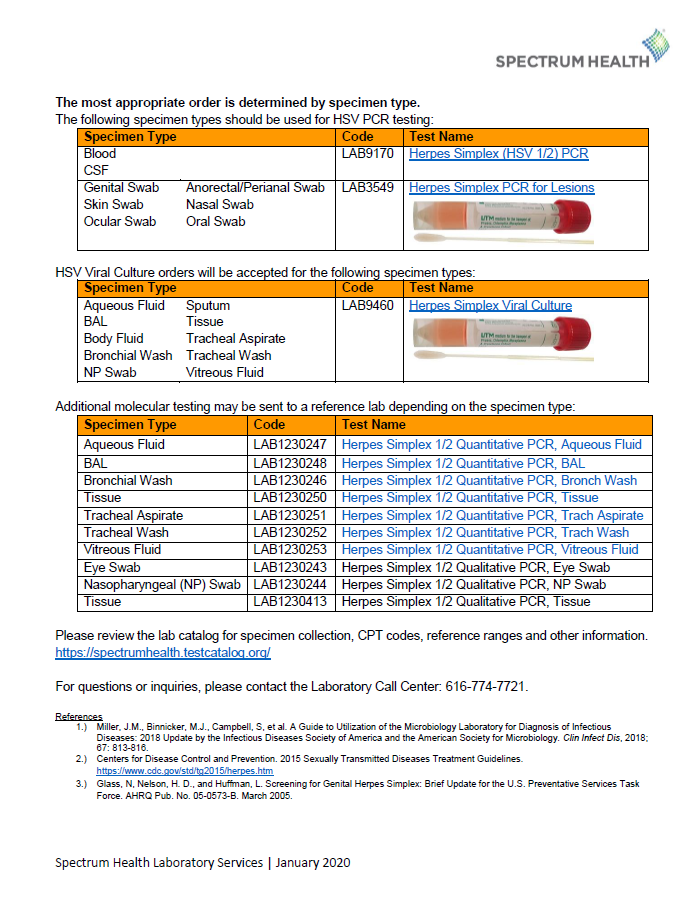
REMINDER: Herpes Simplex Virus (HSV) PCR and HSV Culture Orders
As of January 13, 2020, the Spectrum Health Microbiology Laboratory will switch any viral culture orders placed on cutaneous or mucocutaneous lesion specimens to molecular PCR testing as the preferred diagnostic method.
Please refer to these documents for specimen collection information and appropriate ordering codes:
Appropriate Thyroid Peroxidase Antibody Ordering
The most conclusive evidence for using thyroid peroxidase antibody (TPO) is predictive in nature when evaluating possible subclinical hypothyroidism. If this test is positive, hypothyroidism occurs at a rate of 4.3% per year versus 2.6% per year when the antibody is negative. While this scenario does not cover all clinical indications for ordering TPO, there is no definitive evidence that repeat TPO testing provides additional information.1
Based on this information the ordering of TPO within Spectrum Health is being modified. If the test is ordered more than once on a patient, a screen will appear in EPIC indicating the following: “This test should typically only be resulted once per lifetime. The duplicate checking indicates that this patient has already had this testing performed. Please see chart review for results.” This is not a “hard stop” but providers will need to click “Continue” to proceed with the order.