Effective August 26, 2024, Corewell Health Laboratories West will be updating their High-Risk HPV testing. The new assay will be performed on the Abbott Alinity m at Corewell Health Reference Laboratory West, Grand Rapids. Testing will now automatically include HPV Genotype results whenever HPV is detected. There will no longer be a separate charge to the patient for HPV Genotype testing.
There will also be an expanded number of High-Risk HPV Genotypes reported:
- HPV Genotype 16
- HPV Genotype 18
- HPV Genotype 45
- HPV Genotype 31/33/52/58
- HPV Genotype 35/39/51/56/59/66/68
The new assay is FDA approved for Primary screening in addition to co-testing.
Post hysterectomy vaginal specimens will still be a send out test (LAB1230722).
Below is the new testing algorithm:
PAP HPV Testing Options for Women’s Health v24.2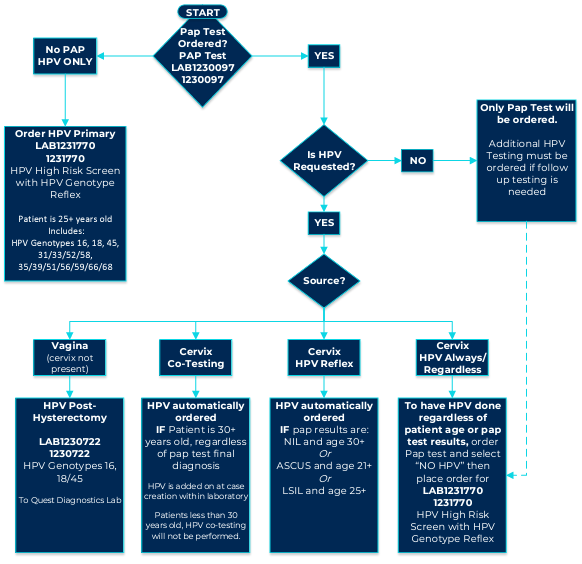
Along with the updated HPV and Genotype assay, HPV results will now be reported simultaneously with PAP testing.
Any STI testing for Chlamydia, Gonococcus, or Trichomonas ordered concurrently on the Thin Prep collection will be reported separately when testing is completed.
TEST INFORMATION:
New Test:
- HPV High Risk Screen with HPV Genotype Reflex [LAB1231770]
- HPV Genotypes 16, 18/45 to Quest Diagnostics Lab [LAB1230722]
Discontinued Test:
- Human Papillomavirus (HPV) DNA Detection with Genotyping, High-Risk Types by PCR, ThinPrep, Varies to Mayo Clinic Laboratories [LAB1230723]
- HPV (Human Papillomavirus) High Risk Screen [LAB263]
- Human Papilloma Virus (HPV) Type 16 and 18/45 mRNA test [LAB3340]
No Change:
- Pap Test [LAB1230097]