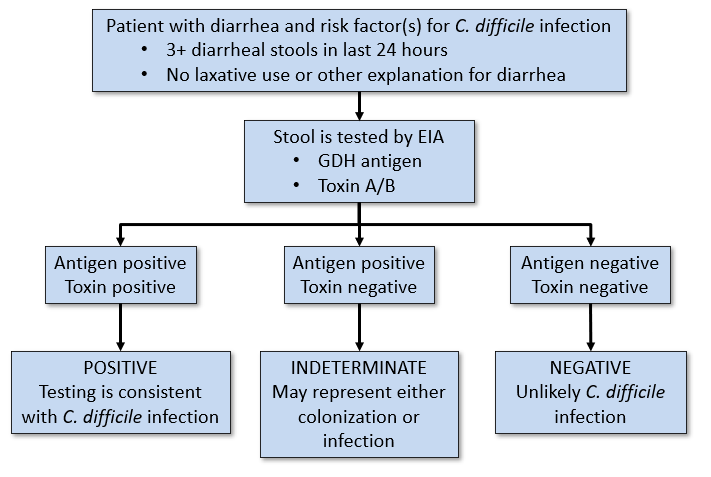
As of March 21, 2022, Spectrum Health Laboratories will adopt a new C. difficile testing approach that no longer reflexes to PCR testing for indeterminate toxin enzyme immunoassay (EIA) results. Rather, PCR testing will only be available upon consultation with Infectious Disease.
C. difficile laboratory diagnosis is complicated by the fact that around 20% of hospitalized adults are colonized with this organism in the absence of symptoms. Diarrhea is common in healthcare settings and can have many causes, so diagnostic tools are challenged to distinguish C. difficile colonization from active infection. The prior testing approach involved performing an EIA for the presence of C. difficile antigen and toxin with reflex to PCR when indeterminate (i.e. antigen positive, toxin negative). Because patients with PCR-positive, toxin EIA-negative results have similar clinical outcomes to those without C. difficile infection, the American College of Gastroenterology updated their clinical guidelines in 2021 to include a testing approach using an antigen/toxin EIA, but without an automatic reflex to PCR. Treatment decisions should be made based on clinical assessment, and treatment should not be withheld based on a negative lab result alone when there is high clinical suspicion.
References:
- Kelly CR, Fischer M, Allegretti JR, et al. ACG Clinical Guidelines: Prevention, Diagnosis, and Treatment of Clostridioides difficile Infections. Am J Gastroenterol. 2021 Jun 1;116(6):1124-1147.
- McDonald LC, Gerding DN, Johnson S, et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018 Mar 19;66(7):987-994.