On June 27th, the name of the current order “Respiratory (non-sputum) Culture without Gram stain” will change to “Throat Culture Comprehensive.” All other specimen collection details and culture workup will remain the same. Given the fact that there are currently several similarly named tests, this change is being made to reduce confusion regarding test selection and to clarify acceptable specimen types.
June 2017
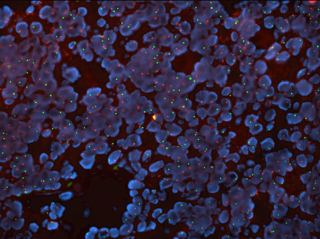
Lung Cancer Mutation Analysis Panel provides targeted treatments for patients
The treatment of non-small cell lung adenocarcinoma has become more successful due to targeted treatments based on the molecular profile of a patient’s particular tumor. The Lung Cancer Mutation Analysis panel is now available for clinical testing at the Spectrum Health Advanced Technology Laboratories (ATL).
All specimens submitted to Spectrum Health Laboratory for testing must be appropriately labeled to assure positive identification and optimum integrity of specimens. In accordance with standards issued by The Joint Commission, at least 2 patient identifiers should be used when providing care or treatment of services (National Patient Safety Goal, NSPG.01.01.01). If 2 patient identifiers are not used on the specimen, the specimen will be rejected, the order will be cancelled and a request for recollection will be made. This includes Gyn Cytology (i.e. Pap) specimens.
What are acceptable Patient Identifiers?
- Patient Full Legal Name
- Date of Birth
- Spectrum Health Medical Record Number (MRN)
Follow these steps for loading your outdoor lockbox during the summer months to prevent overheating of refrigerated and ambient specimens:
1. Place a frozen cool pack in the bottom of the lockbox.
2. Layer 3 – 4 paper towels over the cool pack for insulation.
3. Place refrigerated temperature specimens on top of the paper towels.
4. Layer 3 – 4 paper towels over the refrigerated specimens for insulation.
5. Place ambient temperature specimens on top of the paper towels.
Frozen specimen should not be left in the lockbox for after-hours pickup. These tests may be better preserved in the office freezer until the next day’s courier pickup.
Spectrum Health Laboratory is committed to specimen integrity. The specimen transport integrity guide is available in the online test catalog. If you have further questions please contact Laboratory Services at 616.774.7721.