Written by Yasel Fleitas Alvarez, Ph.D., Chemistry Clinical Advisor, Corewell Health Reference Laboratory West, Michigan Pathology Specialists.
This January we are celebrating the National Thyroid Awareness Month. In United States of America, it is estimated that approximately 20 million people have thyroid disease and most importantly, according to the American Thyroid Association (ATA) as many as 60% of people suffering from a thyroid disorder are not aware they have it.The thyroid is a butterfly shaped-gland located at the front of the neck that produces and release thyroid hormones (See Figure 1).
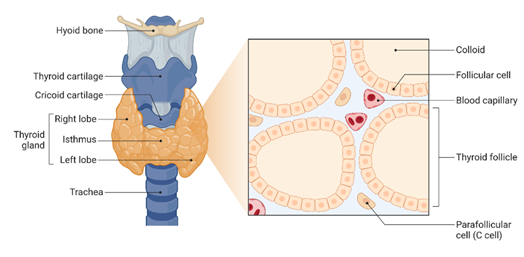
Figure 1. Thyroid Gland Anatomy and Histology
It regulates important physiological functions as:
-
- Breathing
- Heart rate
- Energy production
- Muscle strength
- Body temperature
- Weight
- Mood
Thyroid disease can present in two main forms:
- Hypothyroidism (under functioning thyroid)
- Hyperthyroidism (over functioning thyroid)
Confirmation or exclusion of thyroid disease requires a clinical examination combined with biochemical determination of thyroid hormones (TH) and thyrotropin (TSH)concentrations.
In this blog we discuss the best practices for ordering thyroid function tests for the initial screening of thyroid disease at Corewell Health.

