The following information was updated in the Lab Catalog.
All changes are for Corewell Health Laboratories West.
The following information was updated in the Lab Catalog.
All changes are for Corewell Health Laboratories West.
This issue is resolved. It was identified as an issue within Corewell Health Epic Preference Lists. If you are still encountering issues, please reach out to Service Now. This post will be archived in 7 days.
Due to an error from our system changes that happened on Sunday, March 17, 2024, the HPV reflex on the Pap testing orderable is not reflexing appropriately based on ASCCP Guidelines.
The Cytology Laboratory (Corewell Health Reference Laboratory West) and our Digital Services teams are aware of the error and working on a solution to correct the missed HPVs that were processed this week. As the correction may cause a slight delay in HPV results, please hold add-on requests for HPV until Friday (3/29). If you are concerned about an HPV being missed after this date (3/29/24), please fill out an Add-On form and fax to the number on the form (Note: Pap Specimens are held for 30 days).
We apologize for any inconvenience and confusion. If you have any further questions or concerns, please use the contact us link above.
The following information was updated in the Lab Catalog.
Effective Tuesday, February 20, 2024, the policy for home collected specimen drop offs for Outpatient Laboratories (“draw sites”) has been updated.
For all Corewell Health West Michigan locations: after registration, patients or their designated person will verify the specimen at the laboratory. Laboratory team members will verify the specimen is properly labeled and ensure they have everything needed to be able to test the specimen before the patient or their designated person leaves the building. This does not include specimens collected by a home health nurse or provider.
This will standardize our patient flow when arriving with a home collected specimen and will reduce the need for recollection due to missing information or improper collection.
When specimen collection orders are placed, please notify patients of the change to this process.
RELATED RESOURCES
The following information was updated in the Lab Catalog.
All changes are for Corewell Health Laboratories West.
Written by Yasel Fleitas Alvarez, Ph.D., Chemistry Clinical Advisor, Corewell Health Reference Laboratory West, Michigan Pathology Specialists.
This January we are celebrating the National Thyroid Awareness Month. In United States of America, it is estimated that approximately 20 million people have thyroid disease and most importantly, according to the American Thyroid Association (ATA) as many as 60% of people suffering from a thyroid disorder are not aware they have it.The thyroid is a butterfly shaped-gland located at the front of the neck that produces and release thyroid hormones (See Figure 1).
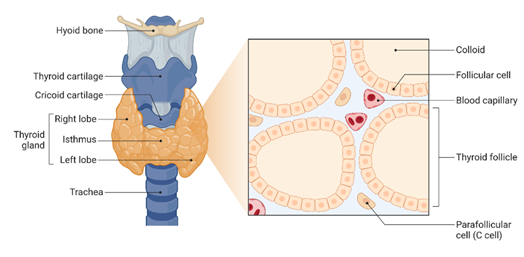
Figure 1. Thyroid Gland Anatomy and Histology
It regulates important physiological functions as:
Thyroid disease can present in two main forms:
Confirmation or exclusion of thyroid disease requires a clinical examination combined with biochemical determination of thyroid hormones (TH) and thyrotropin (TSH)concentrations.
In this blog we discuss the best practices for ordering thyroid function tests for the initial screening of thyroid disease at Corewell Health.
On 10/31/23, a change was made to lab specimen labels, which included a patient’s preferred name on the label, if it is documented in Epic. This highlighted a change in the names of label types seen by staff when reprinting specimen labels in Epic. Due to a mix of Intermec and Zebra printers across the entire Corewell Health system, defaults needed to be removed from Epic build to account for these differences. Users will now be required to manually choose the correct label type when reprinting labels. The Collection Activity behavior should remain the same.
As a reminder, referral testing, tests that are sent to another laboratory to perform, is dependent on the weather. Inclement weather can affect both the turnaround time of testing, and the testing capabilities of the organization. Our referral testing is dependent on outside transportation facilities (such as FedEx and UPS). When those organizations face challenges in weather, our testing capabilities must remain flexible.
If there is inclement weather in and around Memphis, TN (the main FedEx hub), there may be delays and cancellations of testing. We will try our best to communicate these changes as soon as possible via our blog: https://lab.spectrumhealth.org/
For any questions or concerns please “Contact Us” via the link above.
Effective January 17, 2024, UFH Anti-IIa testing will be performed at both Corewell Health Reference Laboratory West and Blodgett Immediate Response Laboratory in Grand Rapids.