NTRK Gene Fusion is Available at Corewell Health West Molecular Diagnostics Laboratory
Molecular Diagnostics
Test Update: Chlamydia and Gonococcus Testing (Alinity m)
Effective October 31, 2022, the Advanced Technology Laboratory’s Molecular Diagnostics Department will accept oropharyngeal and rectal swab specimens as testing sources for Chlamydia and Gonococcus testing using the Alinity m multi-Collect Kit. This specimen type is FDA approved on the Alinity m platform.
NOTE: Specimens collected on patients under the age of 14 or collected for Child Protective Services, will still be sent out to a reference laboratory.
MTHFR C677T [LAB7390], the methylenetetrahydrofolate reductase mutation analysis, is being discontinued as an obsolete test.
The American College of Medical Genetics (ACMG) and American College of Obstetricians and Gynecologists (ACOG) have determined that MTHFR C677T testing has minimal clinical utility. Current testing recommendations indicate that this test should not be ordered as part of a routine evaluation for thrombophilia or adverse pregnancy outcomes.
Effective February 1, 2022, the Cystic Fibrosis (CF) Carrier Screen test, which includes the 23 common variants for cystic fibrosis recommended by ACOG/ACMG, will be discontinued as an orderable test at Spectrum Health Laboratory.
The recommended replacement test is the Cystic Fibrosis Mutation Analysis (Test ID: CFP) offered by Mayo Clinic Laboratories, a 106-variant panel which includes the recommended 23 common variants.
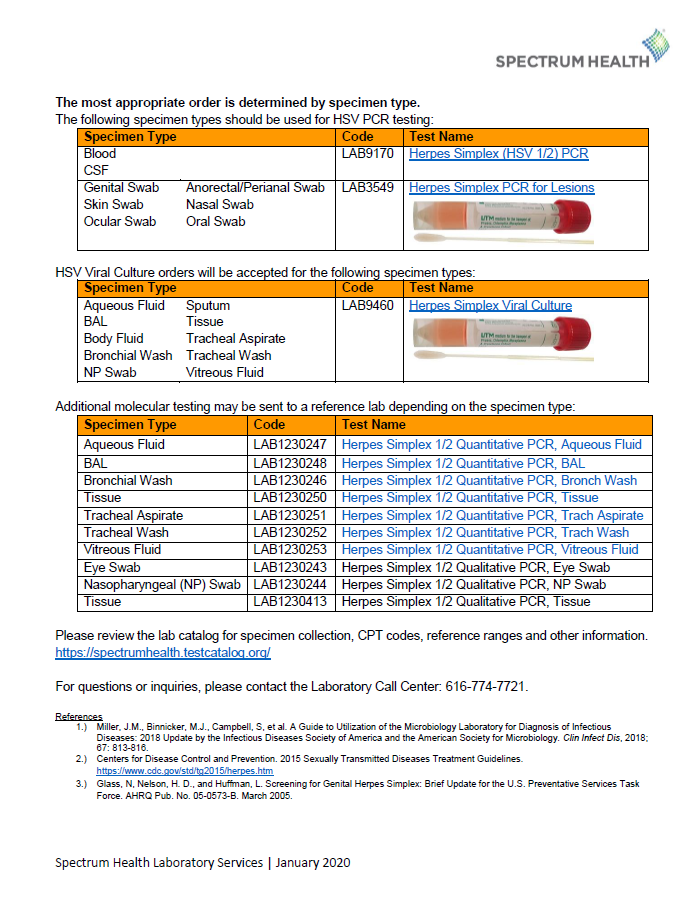
REMINDER: Herpes Simplex Virus (HSV) PCR and HSV Culture Orders
As of January 13, 2020, the Spectrum Health Microbiology Laboratory will switch any viral culture orders placed on cutaneous or mucocutaneous lesion specimens to molecular PCR testing as the preferred diagnostic method.
Please refer to these documents for specimen collection information and appropriate ordering codes:
Herpes Simplex Virus (HSV) IgM Antibody Testing Update
As of November 25th 2019 the following tests for Herpes Simplex Virus (HSV) IgM antibody testing will be obsoleted.
Herpes IgM Antibody Screen – LAB3576
Herpes Simplex Virus (HSV) Antibody Screen, IgM, by EIA, Serum – LAB3578
Herpes Simplex Panel – LAB1230539
Effective Wednesday, November 13, 2019, the new Mycoplasma genitalium (mgen) assay is now available using the Aptima target nucleic acid amplification test (NAAT) for the qualitative detection of ribosomal RNA (rRNA) from Mycoplasma genitalium to aid in the diagnosis of M. genitalium urogenital infections in male and female patients.
Advanced Technology Laboratory plays an important role in Oncology
Spectrum Health Regional Laboratory (SHRL) was featured on EightWest on WOOD TV8 on Tuesday, August 30, 2016. Dr. Stephanie F. Williams, Division Chief of Spectrum Health Adult Bone and Marrow Transplant, and Kim Collison, Director of Laboratory Services, talk about the importance and benefits of having SHRL’s Advanced Technology Laboratory right here in West Michigan.
The Advanced Technology Laboratory is “extremely important, particularly for patients with certain cancers…”
“It helps us to diagnose the patients faster and more accurately; to follow the progress of their disease during the treatments that we give, and even at times to guide us in what is the best treatment for a patient…”
“Within 2 hours we had the answer. We did not have to send that specimen out to another laboratory in another state.”
-Stephanie F. Williams, MD, Division Chief, Spectrum Health Adult Bone and Marrow Transplant